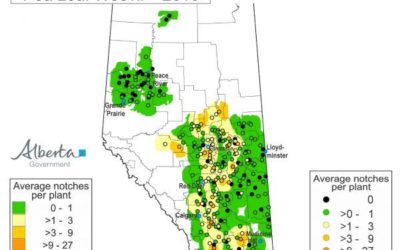
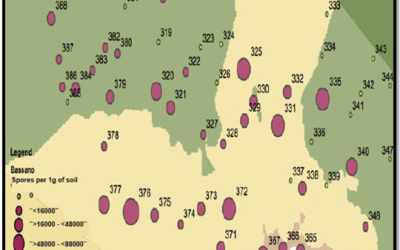
The wet weather last summer in many parts of Alberta has raised concerns about Fusarium head blight (FHB) in this...
Diseases
Pest and Disease Outlook 2020
Top pest and disease concerns in Alberta for the coming year. After a couple of dry years, 2019 was a wetter year in...
Keeping Blackleg at Bay
If blackleg levels are on the rise in your canola field, think about adjusting your tactics for managing this...
The Future of Disease Management in Western Canada
Kelly Turkington is an Agriculture and Agri-Food Canada research scientist and plant pathologist based in Lacombe,...
Fighting Fusarium Head Blight
Two Alberta experts share their views on best practices for managing the fungal disease in cereal crops. Fusarium head...
Sniffing Out Clubroot
It was a sight this past September in canola fields near Brooks in Newell County and again in Leduc County, 2 sniffer...
Clubroot Update (With Recipe For Clubroot Management)
Manitoba Agriculture announced this week it has discovered a clubroot pathotype in South Central Manitoba that is able...
New Clubroot Areas – Scout Your CR Canola
In Saskatchewan, visible clubroot symptoms have been found in two fields in the northwest part of the province in RMs...
Patch Management For Clubroot: You Can Do It! Here’s How
Canola plants yellowing pre-maturely could be infected with clubroot. If you find a patch of canola plants with...
Test Kochia Escapes For Glyphosate Resistance
Glyphosate-resistant kochia is found in all three Prairie provinces. Kochia produces 15,000 to 25,000 seeds per plant,...
Be On The Lookout For Late Blight
This disease affects mainly potatoes and tomatoes and continues to be a risk for all solanaceous crops grown in...
Fungicides – Are They Needed Every Year?
“You need to ask yourself some basic questions when estimating the risk of using fungicides,” says Harry Brook, crop...
Don’t Let Fusarium Upset Your Season
Fusarium head blight (FHB) is a fungal disease of cereal crops. When disease infection is severe, FHB can recognized...
Fighting Fusarium Head Blight
Cereal growers in Alberta have a new weapon in the fight against fusarium head blight (FHB). It’s an online risk...
Southern Alberta Clubroot Response Workshop
Clubroot was confirmed in 4 fields southeast of Calgary in the fall of 2018. This meeting will help you gain a better...
Pea Leaf Weevil Risk Map from 2018 Survey Season
The annual pea leaf weevil (PLW) survey that is conducted by the Alberta Insect Pest Monitoring Network was released...
Clubbing Clubroot: An Update On Breeding Clubroot-Resistant Canola
On the Prairies, clubroot appeared in Alberta in 2003, in Saskatchewan in 2008 and Manitoba in 2013. As any grower can...
How to Test Soils for the Clubroot Pathogen
Soil tests for clubroot can have two objectives: 1. Is the clubroot pathogen present? (Yes/No test) This could be done...
Clubroot Identified in Rocky View County Southeast of Calgary
Clubroot has been identified in canola southeast of Calgary. Although clubroot has been found in various counties in...
Clubroot Management: Harvest Theme
Look. During harvest scouting, carefully examine the roots of unhealthy looking plants and also random plants chosen...