Soil tests for clubroot can have two objectives:
1. Is the clubroot pathogen present? (Yes/No test) This could be done with just one composite sample per field. Take samples (scoops) from higher-risk areas, such as field entrances, low spots or a water run that may bring resting spores into the field. For more refined results, send individual samples from each ‘hot spot’ to better understand the distribution of the pathogen within a field.
Farmers can use a ‘yes’ result to confirm that they should grow clubroot-resistant (CR) varieties. The general recommendation is to use CR varieties as soon as clubroot is found in the area (which are now most areas of the Prairies). A ‘yes’ result should also encourage heightened biosecurity and sanitation measures to keep clubroot from spreading within that field and to other fields.
Experience has shown that a positive result may not immediately lead to noticeable levels of clubroot. Resting spore levels could be too low for infection to be visible. Environmental conditions may not be conducive. Spores may not be viable. But soil tests can complement plant scouting practices for early detection of clubroot.
As a winter project, farmers could save the buckets of sampled soil and use it to grow canola plants to see if they produce galls. Remember, clubroot prefers moist and warm soil so keep the soil wet, but not saturated, and in a warm environment.
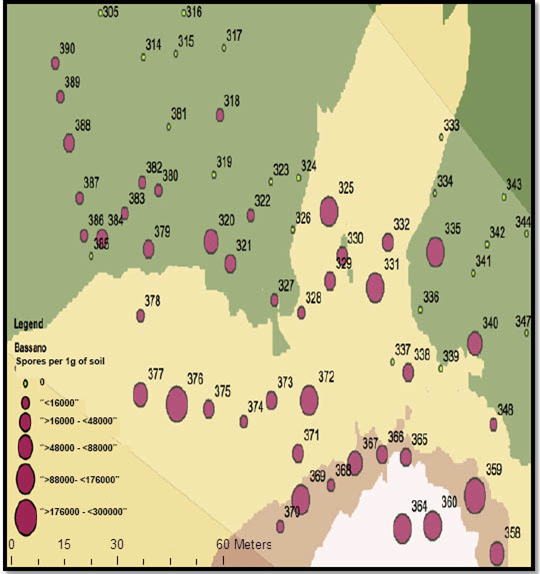
2. What is the resting spore count per gram of soil? (Quantitative test) In addition to the yes/no test, labs can also measure the amount of resting spores per gram of soil. Sample to a 15cm depth as spores in the top layer are most likely to cause yield-limiting infection. Quantitative results could be used to assess the clubroot risk level, but the ‘grey-area’ nature of this test creates problems for interpretation. Fewer spores mean lower risk, but clubroot infection can still occur at 1,000 spores per gram of soil, or less. If one field has 10,000 spores per gram and the neighbouring field has 100,000, both fields have a problem. And if a test shows 1,000,000 spores per gram, the field is clearly at risk and symptoms are likely to appear under most conditions.
Adding to the grey area is that results can be different depending on the lab (results cannot be compared lab to lab because of their different sampling, storage, extraction and analysis protocols) and the soil sampling location.
When sampling to determine the spore load in a heavily-infested area, collect samples from only that area as a composite sample from other areas may dilute the spore concentration. However, to get a fair estimate of spore load within a heavily-infested area, include soil from the canola row and from between the rows.
Eventually other objectives may be added – How many spores are actually viable and alive? What pathotypes are present and at what numbers? – which could be used to choose a variety with resistance to the predominant pathotypes in a field. This test for differing pathotypes, similar to the blackleg system, is not ready but significant progress has been made.
Source: Canola Watch