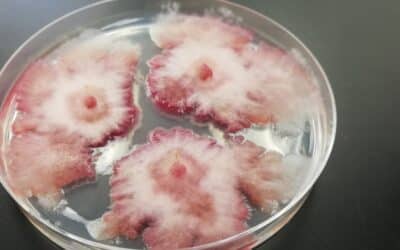
Alberta farmers only need to look at the history of their fields and weather from years past to predict what pests and...
Diseases
In 2024, Canola Disease Threats are Lurking in the Soil
PHOTO: Clubroot. Alberta farmers only need to look at the history of their fields and weather from years past to...
Newer Cereal Crop Diseases on the Rise for 2024
PHOTO: Bacterial leaf streak. Alberta farmers only need to look at the history of their fields and weather from years...
Bacterial Leaf Streak — An Increasing Problem in Alberta
With seed tests now available and awareness on the rise, bacterial leaf streak is a growing problem in Alberta. Cereal...
New Corteva Cereal Fungicide Seed Treatment Straxan Launches
Straxan fungicide seed treatment, a seed and soil-borne cereal disease treatment, has commercially launched in Canada,...
Stay Vigilant in Continued Fight Against Fusarium
Information and awareness have always been and continue to be the best defences against fusarium. While fusarium may...
New Clubroot Strains Capable of Infecting Resistant Canola Found
A study from the University of Alberta has identified new strains of clubroot across Western Canada, an Aug. 14 news...
RDAR Makes $1.25 Million Investment in Clubroot Research
Results Driven Agriculture Research (RDAR), the Alberta Canola Producers Commission and the Saskatchewan Canola...
Using Silicon in Soil to Battle Clubroot
A new study from the University of Alberta has found that by adding silicon to soil it could help battle clubroot, a...
Alberta Fusarium Levels in Seed Low for 2023
The 2023 Fusarium Seed Infection Surveillance for Alberta has found low levels of fusarium infected seed in the...
Wide Range of Pests, Diseases for 2023 Crops in Alberta
Farmers in Alberta may face a range of issues in their fields with pests and crop diseases for the 2023 growing...
Fusarium is Becoming the Fungus Among Us
There’s reason to believe Fusarium could become a negligible problem. It’s been two years since an important reform...
Alberta Crop Disease Outlook for the 2022 Growing Season
Your province wide crop disease outlook for the 2022 growing season. Future outlooks for any industry can be tricky....
A Total Fusarium Management Strategy for Alberta
An update on where Alberta is at in its new approach to the fusarium fight. Farming small grains, like other...
Clubroot Should be Top of Mind for Every Canola Grower
The fight against clubroot has evolved over the past few decades since it was discovered in Alberta. A lot has changed...
Latest Alberta Fusarium Seed Infection Map Released
A new map showing fusarium seed infection in Alberta between Sept. 1, 2020 and April 30, 2021 has been released by the...
Rotten Roots
Plants early season in the season infected with Aphanomyces. Photo: Sherrilyn Phelps, Saskatchewan Pulse Growers Root...
Fusarium Seed Infection Map Released
A map showing Fusarium graminearum seed infection in Alberta between Sept. 1 to Dec. 31, 2020 has been released by the...
Clubroot Resistance Breakdown Happening
Nine new strains of clubroot have been discovered in Western Canadian fields, meaning genetic resistance to the canola...
Moving Forward with Fusarium Regulations Eased
With fusarium removed from Alberta’s Pest and Nuisance Control Regulations, the seed industry is looking forward to a...